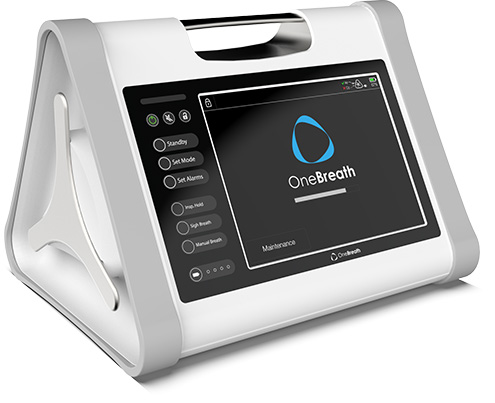
OneBreath represents a paradigm shift in mechanical ventilation with its unmatched affordability and breadth of capabilities.
Product Datasheet
Click the button below to view the OneBreath ventilator data sheet.
How it’s Made
OneBreath works with world-class manufacturing partners to ensure our ventilators are built to FDA standards using a robust and redundant global supply chain.
Where it’s Sold
OneBreath will be available wherever FDA clearance is accepted. Our primary markets in India and other parts of South Asia will open first.